(COVID-19) IgM/IgG Antibody Rapid test kit (Latex Chromatography)
Intended Use
It is for rapid,qualitative detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG/IgM antibody in human whole blood, serum or plasma sample. The test is to be used as an aid in the diagnosis of coronavirus infection disease, which is caused by SARS-CoV-2. The test provides preliminary test results. Negative results don’t preclude SARS-CoV-2 infection and they cannot be used as the sole basis for treatment or other management decision. For in vitro diagnostic use only.
Test Principle
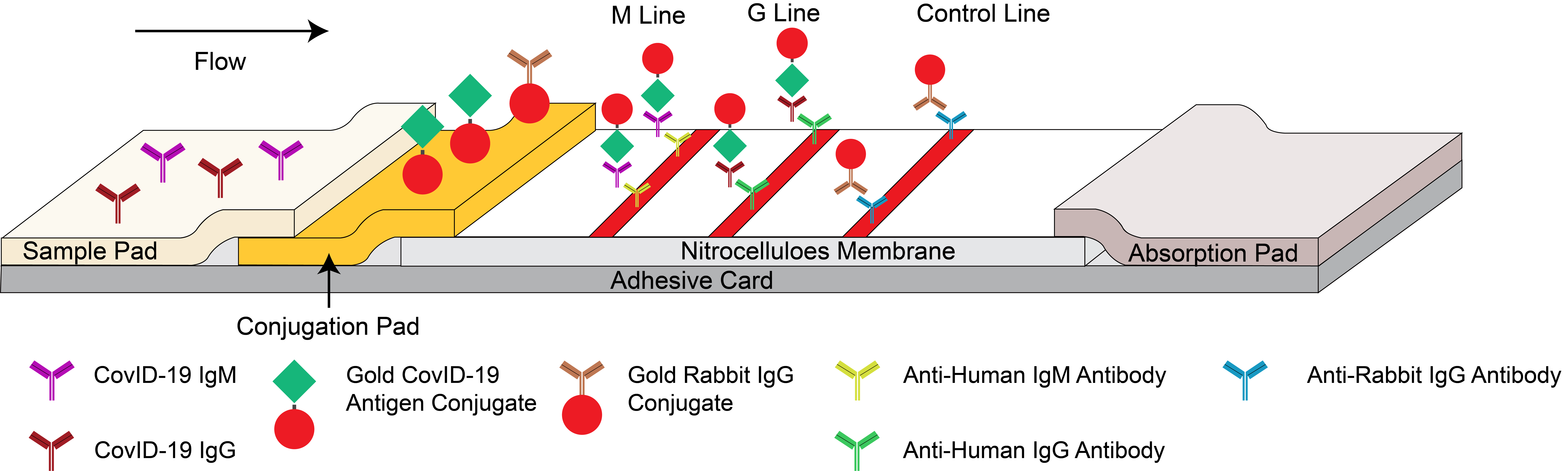
It is based on the principle of capture immunoassay for determination of COVID-19 IgG/IgM antibodies in human whole blood, serum and plasma. When the sample is added to the test device, the sample will be absorbed into the device by capillary action, mixing with SARS-CoV-2 recombinant antigen-color latex conjugate and flowing through the pre-coated membrane.
Main Contents
Components provided are listed in the table.
| Component REF REF | B001C-01 | B001C-25 |
| Test Cassette | 1 test | 25 tests |
| Disposable | 1 piece | 25 pcs |
| Sample Lysis Solution | 1 tube | 25 tubes |
| Instructions For Use | 1 piece | 1 piece |
| Certificate of Conformity | 1 piece | 1 piece |
Operation Flow
If the reagent is stored in a refrigerator at 4-8℃, remove the reagent card and uilibrate at room temperature for more than 30 minutes.
1. Open the inspection card aluminum foil bag. Remove the test card and place it horizontally on a table.
2. Use pipette to aspirate sample (serum, plasma or whole blood) and add 10μL to the sample hole of the test card, and then add 60μL sample dilution solution immediately. Start counting.
3. 15 mins later, read the results visually. (Note: do NOT read the results after 20 mins!)
Result Interpretation
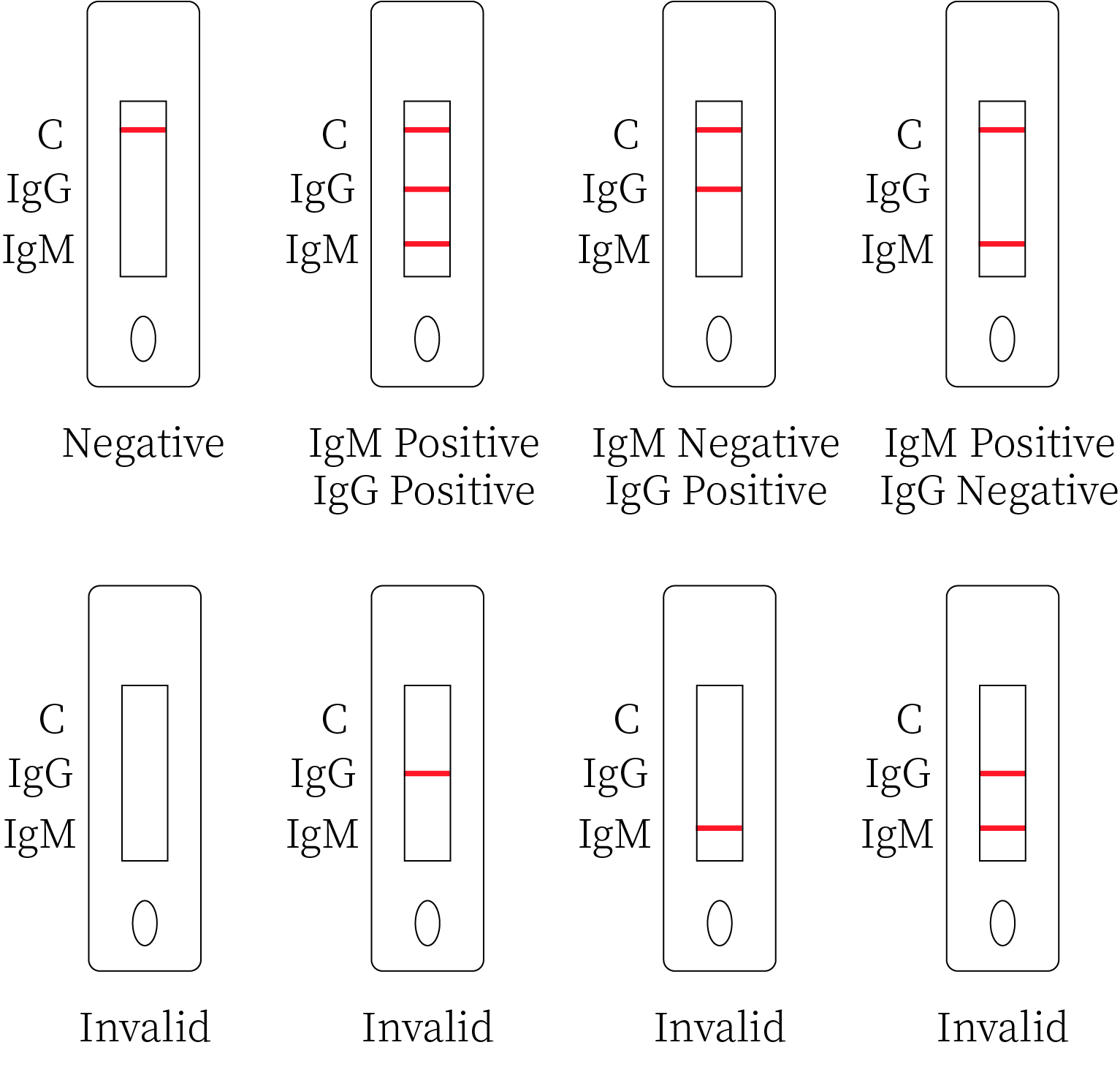
1. Negative result
If only the quality control line C appears and the detection lines G and M do not show, it means that no novel coronavirus antibody is detected and the result is negative.
2. Positive Result
2.1 If both the quality control line C and the detection line M appear, it means that the novel coronavirus IgM antibody is detected, and the result is positive for the IgM antibody.
2.2 If both the quality control line C and the detection line G appear, it means that the novel coronavirus IgG antibody is detected and the result is positive for the IgG antibody.
2.3 If both the quality control line C and the detection lines G and M appear, it means that the novel coronavirus IgG and IgM antibodies are detected, and the result is positive for both IgG and IgM antibodies.
3. Invalid Result
If the quality control line C cannot be observed, results will be invalid regardless of whether a test line shows, and the test should be repeated.
Order Information
| Product Name | Cat. No | Size | Specimen | Shelf Life | Trans. & Sto. Temp. |
| (COVID-19) IgM/IgG Antibody Rapid test kit (Latex Chromatography) | B001C-01 | 1test/kit | Serum/Plasma/Whole Blood | 18 Months | 2-30℃ / 36-86℉ |
| B001C-01 | 25 tests/kit |