COVID-19/Flu A&B Rapid Immunoassay for Direct Detection
COVID-19/Flu A&B Rapid Immunoassay for Direct Detection,
COVID-19/Flu A&B Rapid Immunoassay for Direct Detection,
Product details
Intended Use
SARS-CoV-2 & Influenza A/B Antigen Combo Rapid Test Kit (Lateral chromatography) is to be used in conjunction with clinical manifestations and other laboratory test results to assist in the diagnosis of patients with suspected SARS-CoV-2 or Influenza A/B infection. The test is only to be used by medical professionals. It provides only an initial screening test result and more specific alternative diagnosis methods should be performed in order to obtain the confirmation of SARS-CoV-2 or Influenza A/B infection. For professional use only.
Test Principle
SARS-CoV-2 & Influenza A/B Antigen Combo Rapid Test Kit (Lateral chromatography) is a lateral flow chromatographic immunoassay. It has two results Windows. On the left for SARS-CoV-2 antigens. It has two pre-coated lines, “T” Test line and “C” Control line on the nitrocellulose membrane. On the right is the result window of FluA/FluB, it has three pre-coated lines, “T1” FluA Test line, “T2” FluB Test line and “C” Control line on the nitrocellulose membrane.
Main Contents
Components provided are listed in the table.
| Product Name | Cat. No | Size | Specimen | Shelf Life | Trans. & Sto. Temp. |
|
SARS-Cov-2 & Influenza A&B Antigen Rapid Test Kit(Immunochromatographic Assay) |
B005C-01 | 1test/kit | Nasalpharyngeal Swab, Oropharyngeal Swab | 24 Months | 2-30℃ / 36-86℉ |
| B005C-05 | 5 tests/kit | ||||
| B005C-25 | 25 tests/kit |
Operation Flow
- Step 1: Sampling
Tilt patient’s head back 70 degrees. Carefully insert the swab into nostril until the swab reaches the back of the nose. Leave swab in each nostril for 5 seconds to absorb secretions.
- Step 2: Testing

1. Remove an extraction tube from the kit and a test box from the film bag by tearing the notch. Put them on the horizontal plane.
2. After sampling, soak the smear below the liquid level of the sample extraction buffer, rotate and press 5 times. Immerse time of smear at least 15s.
3. Remove the swab and press the edge of the tube to squeeze out the liquid in the swab. Throw the swab into the biological hazardous waste.
4. Fix the pipette cover firmly on the top of the suction tube. Then gently turn the extraction tube5 times.
5. Transfer 2 to 3 drops (about 100 ul) of the sample to the sample surface of the test band and start the timer. Note: if frozen samples are used, the samples must have room temperature.
- Step 3: Reading
15 mins later, read the results visually. (Note: do NOT read the results after 20 mins!)
Result Interpretation
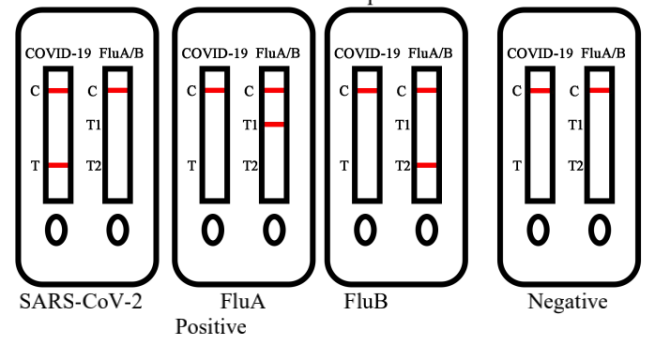
1.SARS-CoV-2 Positive Result
Colored bands appear at both test line (T) and control line (C). It indicates a
positive result for the SARS-CoV-2 antigens in the specimen.
2.FluA Positive Result
Colored bands appear at both test line (T1) and control line (C). It indicatesa
positive result for the FluA antigens in the specimen.
3.FluB Positive Result
Colored bands appear at both test line (T2) and control line (C). It indicatesa
positive result for the FluB antigens in the specimen.
4.Negative Result
Colored band appear at control line (C) only. It indicates that the
concentration of the SARS-CoV-2 and FluA/FluB antigens do not exist or
below the detection limit of the test.
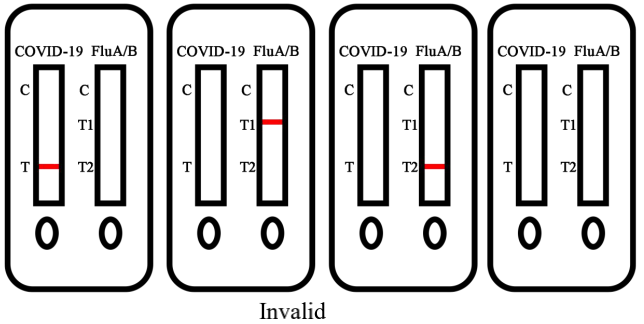
5.Invalid Result
No visible colored band appears at control line after performing the test. The
directions may not have been followed correctly or the test may have
deteriorated. It is recommended that the specimen be re-tested.
Order Information
| Product Name | Cat. No | Size | Specimen | Shelf Life | Trans. & Sto. Temp. |
| SARS-CoV-2 & Influenza A/B Antigen Combo Rapid test kit (Lateral chromatography) | B005C-01 | 1test/kit | Nasalpharyngeal Swab | 18 Months | 2-30℃ / 36-86℉ |
| B005C-05 | 5 tests/kit | ||||
| B005C-25 | 25 tests/kit |
COVID-19/Flu A&B test is a lateral flow immunoassay intended for the in vitro rapid, simultaneous qualitative
detection and differentiation of nucleocapsid antigen from SARS-CoV-2, influenza A and/or influenza B directly from anterior
nasal or nasopharyngeal swab specimens obtained from individuals, who are suspected of respiratory viral infection
consistent with COVID-19 by their healthcare provider, within the first five days of onset of symptoms. Clinical signs and
symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar. Testing is limited to laboratories
certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the
requirements to perform moderate, high, or waived complexity tests. This product is authorized for use at the Point of Care
(POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of
Accreditation.