Leishmania IgG/IgM Antibody Test Kit (Immunochromatographic Assay) – Bioantibody
Leishmania IgG/IgM Antibody Test Kit (Immunochromatographic Assay) – Bioantibody Detail:
Product details
Intended Use
This product is suitable for the qualitative clinical screening of serum/plasma/whole blood samples for the detection of antibodies against Leishmania. It is a simple, rapid and non-instrumental test for the diagnosis of kala-azar caused by Leishmania.
Test Principle
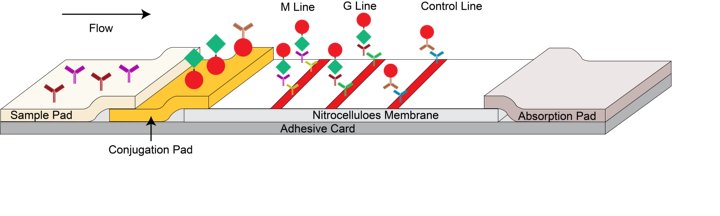
This product is a lateral flow chromatographic immunoassay. The test cassette consists of: 1) a burgundy colored conjugate pad containing recombinant rK39 antigen conjugated with colloid gold (Leishmania conjugates) and rabbit IgG-gold conjugates; 2) a nitrocellulose membrane strip containing two test bands (M and G bands) and a control band (C band).
Main Contents
Components provided are listed in the table.
| Materials / provided | Quantity (1 Test/Kit) | Quantity(5Tests/Kit) | Quantity(25Tests/Kit) |
| Test Kit | 1 test | 5 tests | 25 tests |
| Buffer | 1 bottle | 5 bottles | 25/2 bottles |
| Dropper | 1 piece | 5 pcs | 25 pcs |
| Specimen Transport Bag | 1 piece | 5 pcs | 25 pcs |
| Disposable Lancet | 1 piece | 5 pcs | 25 pcs |
| Instructions For Use | 1 piece | 1 piece | 1 piece |
| Certificate of Conformity | 1 piece | 1 piece | 1 piece |
Operation Flow
Step 1: Sampling
Collect human Serum/Plasma/Whole blood properly.
Step 2: Testing
Please read the instructions carefully before testing. Before testing, allow the test kit, sample solution and sample to be balanced to temperature (15-30℃or 59-86 degrees Fahrenheit).
1.Remove an extraction tube from the kit and a test box from the film bag by tearing the notch. Put them on the horizontal plane.
2.Open the inspection card aluminum foil bag. Remove the test card and place it horizontally on a table.
3.Use a disposable pipette, transfer one drop (about 20μL) fingertip blood/or 4μL serum /or 4μL plasma/ or 4μL whole blood into the sample well on the test cassette.
4.Open the buffer tube. Put 3 drops (about 80 μL) of assay diluent into the assay diluent well round shaped. Start counting.
Step 3: Reading
Read the result at 5-10 mins. Results after 10 minutes are invalid.
Result Interpretation
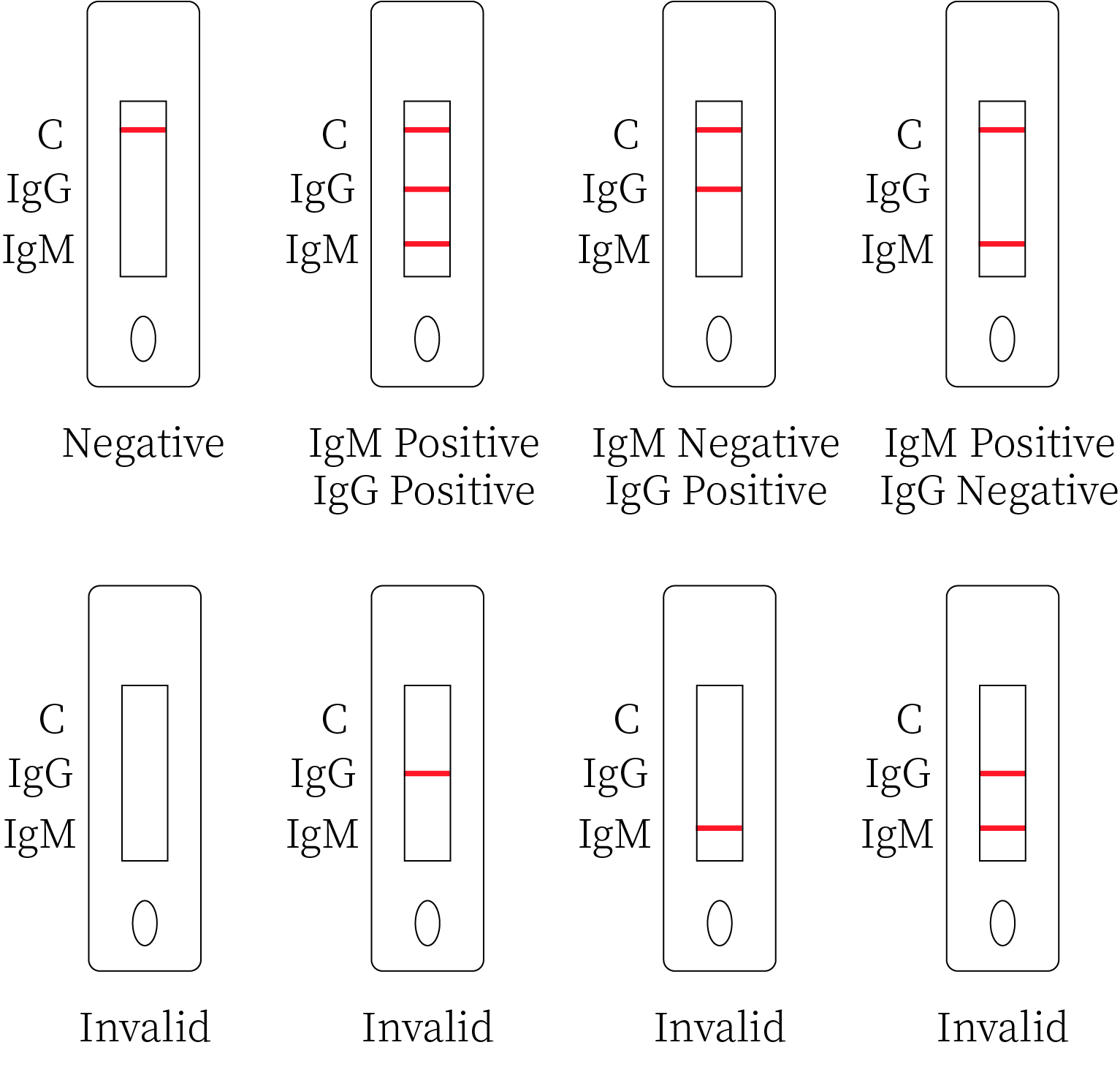
Negative result
If only the quality control line C appears and the detection lines G and M do not show
Positive Result
1.Both the quality control line C and the detection line M appear= the Leishmania IgM antibody is detected, and the result is positive for the IgM antibody.
2.Both the quality control line C and the detection line G appear= the Leishmania IgG antibody is detected and the result is positive for the IgG antibody.
3.Both the quality control line C and the detection lines G and M appear=the Leishmania IgG and IgM antibodies. are detected, and the result is positive for both IgG and IgM antibodies.
Invalid Result
The quality control line C cannot be observed, results will be invalid regardless of whether a test line shows, and the test should be repeated.
Order Information
| Product Name | Cat. No | Size | Specimen | Shelf Life | Trans. & Sto. Temp. |
|
Leishmania IgG/IgM Antibody Test Kit (Immunochromatographic Assay) |
B020C-01 | 1test/kit | Serum/Plasma/Whole Blood | 18 Months | 2-30℃ / 36-86℉ |
| B020C-05 | 5 tests/kit | ||||
| B020C-25 | 25 tests/kit |
Product detail pictures:

Related Product Guide:
In an effort to provide you advantage and enlarge our business enterprise, we even have inspectors in QC Staff and assure you our greatest provider and item for Leishmania IgG/IgM Antibody Test Kit (Immunochromatographic Assay) – Bioantibody , The product will supply to all over the world, such as: Hungary, Kuwait, Orlando, Looking forward, we will keep pace with the times, continuing to create new products. With our strong research team, advanced production facilities, scientific management and top services, we will supply high quality products to our customers worldwide. We sincerely invite you to be our business partners for mutual benefits.
Cooperate with you every time is very successful, very happy. Hope that we can have more cooperation!