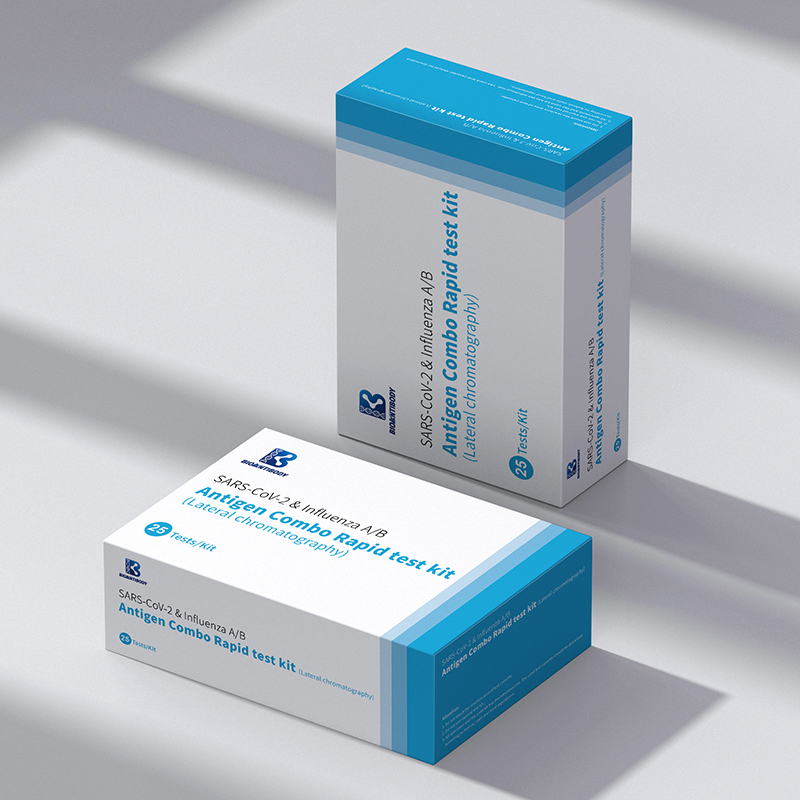
One of Hottest for China New Product! in Vitro Diagnostic Reagent (IVD Reagents) & Influenza a+B Antigen Combo Test Kit
With our leading technology also as our spirit of innovation,mutual cooperation, benefits and development, we are going to build a prosperous future jointly with your esteemed company for One of Hottest for China New Product! in Vitro Diagnostic Reagent (IVD Reagents) & Influenza a+B Antigen Combo Test Kit, We warmly welcome you to definitely build cooperation and produce a bright long run along with us.
With our leading technology also as our spirit of innovation,mutual cooperation, benefits and development, we are going to build a prosperous future jointly with your esteemed company for China Virus Test, Virus Igg/Igm, Our business activities and processes are engineered to make sure our customers have access to widest range of products with the shortest supply time lines. This achievement is made possible by our highly skilled and experienced team. We look for people who want to grow with us around the globe and stand out from the crowd. We now have people who embrace tomorrow, have vision, love stretching their minds and going far beyond what they thought was achievable.
Product details
Intended Use
SARS-CoV-2 & Influenza A/B Antigen Combo Rapid Test Kit (Lateral chromatography) is to be used in conjunction with clinical manifestations and other laboratory test results to assist in the diagnosis of patients with suspected SARS-CoV-2 or Influenza A/B infection. The test is only to be used by medical professionals. It provides only an initial screening test result and more specific alternative diagnosis methods should be performed in order to obtain the confirmation of SARS-CoV-2 or Influenza A/B infection. For professional use only.
Test Principle
SARS-CoV-2 & Influenza A/B Antigen Combo Rapid Test Kit (Lateral chromatography) is a lateral flow chromatographic immunoassay. It has two results Windows. On the left for SARS-CoV-2 antigens. It has two pre-coated lines, “T” Test line and “C” Control line on the nitrocellulose membrane. On the right is the result window of FluA/FluB, it has three pre-coated lines, “T1” FluA Test line, “T2” FluB Test line and “C” Control line on the nitrocellulose membrane.
Main Contents
Components provided are listed in the table.
| Product Name | Cat. No | Size | Specimen | Shelf Life | Trans. & Sto. Temp. |
|
SARS-Cov-2 & Influenza A&B Antigen Rapid Test Kit(Immunochromatographic Assay) |
B005C-01 | 1test/kit | Nasalpharyngeal Swab, Oropharyngeal Swab | 24 Months | 2-30℃ / 36-86℉ |
| B005C-05 | 5 tests/kit | ||||
| B005C-25 | 25 tests/kit |
Operation Flow
- Step 1: Sampling
Tilt patient’s head back 70 degrees. Carefully insert the swab into nostril until the swab reaches the back of the nose. Leave swab in each nostril for 5 seconds to absorb secretions.
- Step 2: Testing

1. Remove an extraction tube from the kit and a test box from the film bag by tearing the notch. Put them on the horizontal plane.
2. After sampling, soak the smear below the liquid level of the sample extraction buffer, rotate and press 5 times. Immerse time of smear at least 15s.
3. Remove the swab and press the edge of the tube to squeeze out the liquid in the swab. Throw the swab into the biological hazardous waste.
4. Fix the pipette cover firmly on the top of the suction tube. Then gently turn the extraction tube5 times.
5. Transfer 2 to 3 drops (about 100 ul) of the sample to the sample surface of the test band and start the timer. Note: if frozen samples are used, the samples must have room temperature.
- Step 3: Reading
15 mins later, read the results visually. (Note: do NOT read the results after 20 mins!)
Result Interpretation


1.SARS-CoV-2 Positive Result
Colored bands appear at both test line (T) and control line (C). It indicates a
positive result for the SARS-CoV-2 antigens in the specimen.
2.FluA Positive Result
Colored bands appear at both test line (T1) and control line (C). It indicatesa
positive result for the FluA antigens in the specimen.
3.FluB Positive Result
Colored bands appear at both test line (T2) and control line (C). It indicatesa
positive result for the FluB antigens in the specimen.
4.Negative Result
Colored band appear at control line (C) only. It indicates that the
concentration of the SARS-CoV-2 and FluA/FluB antigens do not exist or
below the detection limit of the test.
5.Invalid Result
No visible colored band appears at control line after performing the test. The
directions may not have been followed correctly or the test may have
deteriorated. It is recommended that the specimen be re-tested.
Order Information
| Product Name | Cat. No | Size | Specimen | Shelf Life | Trans. & Sto. Temp. |
| SARS-CoV-2 & Influenza A/B Antigen Combo Rapid test kit (Lateral chromatography) | B005C-01 | 1test/kit | Nasalpharyngeal Swab | 18 Months | 2-30℃ / 36-86℉ |
| B005C-05 | 5 tests/kit | ||||
| B005C-25 | 25 tests/kit |
With our leading technology also as our spirit of innovation,mutual cooperation, benefits and development, we are going to build a prosperous future jointly with your esteemed company for One of Hottest for China New Product! in Vitro Diagnostic Reagent (IVD Reagents) & Influenza a+B Antigen Combo Test Kit, We warmly welcome you to definitely build cooperation and produce a bright long run along with us.
One of Hottest for China Virus Test, Virus Igg/Igm, Our business activities and processes are engineered to make sure our customers have access to widest range of products with the shortest supply time lines. This achievement is made possible by our highly skilled and experienced team. We look for people who want to grow with us around the globe and stand out from the crowd. We now have people who embrace tomorrow, have vision, love stretching their minds and going far beyond what they thought was achievable.