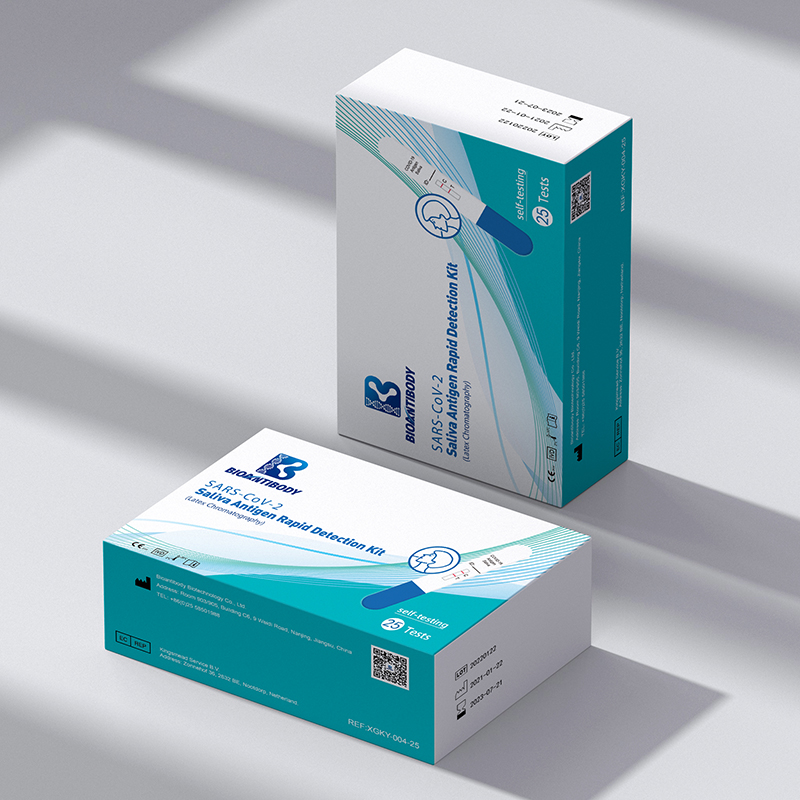
SARS-CoV-2 Saliva Antigen Rapid Detection kit (Latex Chromatography) (Mouth-Type)
Intended Use
SARS-CoV-2 Saliva Antigen Rapid Detection Kit (Latex Chromatography) is to be used in conjunction with clinical
manifestations and other laboratory test results to assist in the diagnosis of patients with suspected SARS CoV-2 infection. The test
is only to be used by medical professionals. It provides only an initial screening test result and more specific alternative diagnosis
methods should be performed in order to obtain the confirmation of SARS-CoV-2 infection. for professional use only.
Test Principle
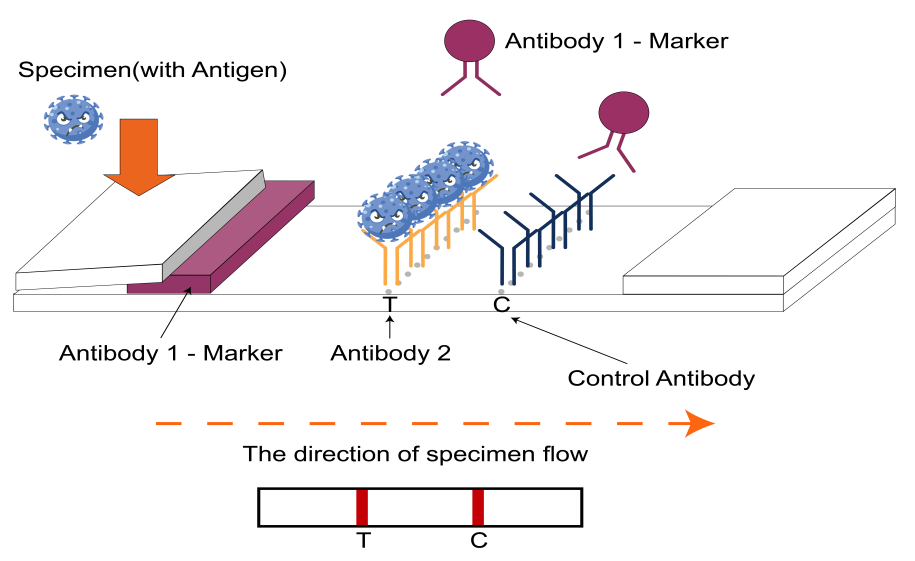
It is a lateral flow assay that qualitatively detects the presence of nucleocapsid (N) protein in upper respiratory samples. This lateral flow assay is designed with the Double-antibody sandwich immunoassay format.
Main Contents
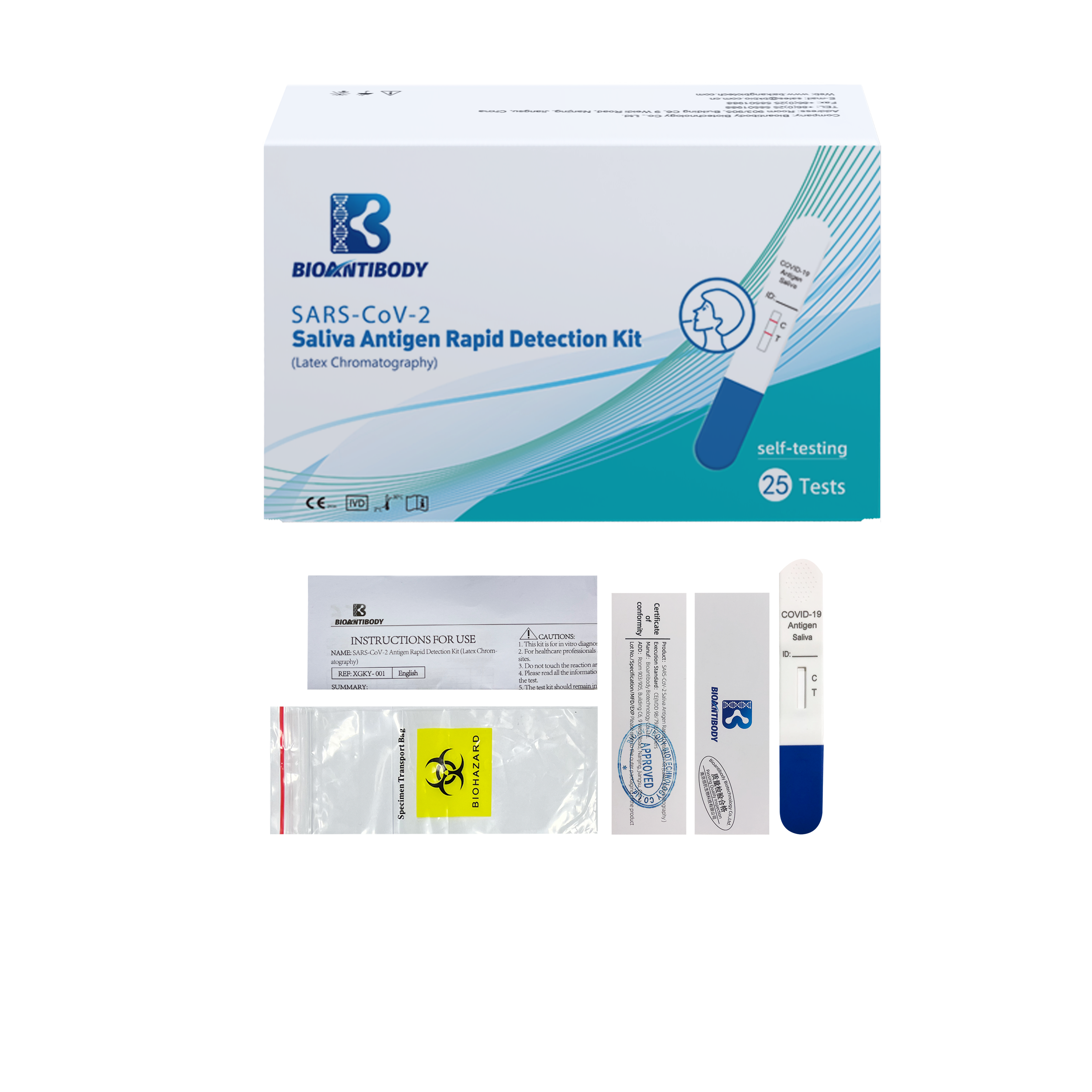
Components provided are listed in the table.
| Component REF /REF | XGKY-003 | XGKY-003-5 | XGKY-003-25 |
| Instructions For Use | 1 piece | 1 piece | 1 piece |
| Certificate of Conformity | 1 piece | 1 piece | 1 piece |
Operation Flow
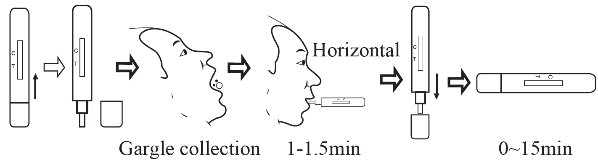
2. Remove the lid and put the cotton core directly under the tongue for two minutes to soak the saliva (Note: Keep your tongue pressed against the wick and don't roll it up). The wick must be immersed in the saliva for two minutes or until the liquid appears in the viewing window of the cassette.
3. After two minutes, remove the kit and close the lid, place it on a flat surface. Start counting.
4. 15 mins later, read the results visually. (Note: do NOT read the results after 20 mins!)
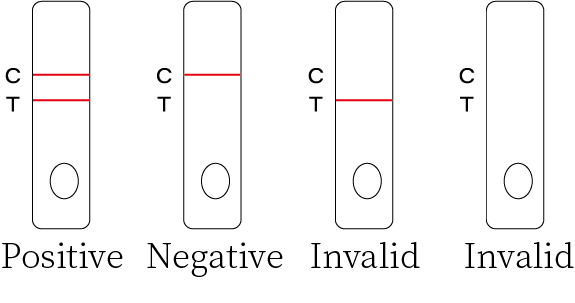
Result Interpretation
Positive Result
Colored bands appear at both test line (T) and control line (C). It indicates apositive result for the SARS-CoV-2 antigens in the specimen.
Negative Result
Colored band appear at control line (C) only. It indicates that the concentration of the SARS-CoV-2 antigens does not exist or below the detection limit of the test.
Invalid Result
No visible colored band appears at control line after performing the test. The
directions may not have been followed correctly or the test may have deteriorated. It is recommended that the specimen be re-tested.
Order Information
| Product Name | Cat. No | Size | Specimen | Shelf Life | Trans. & Sto. Temp. |
| SARS-CoV-2 Saliva Antigen Rapid Detection kit (Latex Chromatography)(Mouth-Type) | XGKY-003 | 1test/kit | Oral Fluid | 18 Months | 2-30℃ / 36-86℉ |
| XGKY-003-5 | 5 tests/kit | ||||
| XGKY-003-25 | 25 tests/kit |