SARS-CoV-2 Saliva Antigen Rapid Detection Kit (Latex Chromatography)
Intended Use
SARS-CoV-2 Saliva Antigen Rapid Detection Kit (Latex Chromatography) is to be used in conjunction with clinical manifestations and other laboratory test results to assist in the diagnosis of patients with suspected SARS-CoV-2 infection. The test
is only to be used by medical professionals. It provides only an initial screening test result and more specific alternative diagnosis methods should be performed in order to obtain the confirmation of SARS-CoV-2 infection. for professional use only.
Test Principle
It is a lateral flow assay that qualitatively detects the presence of nucleocapsid (N) protein in upper respiratory samples. This lateral flow assay is designed with the Double-antibody sandwich immunoassay format.

Main Contents
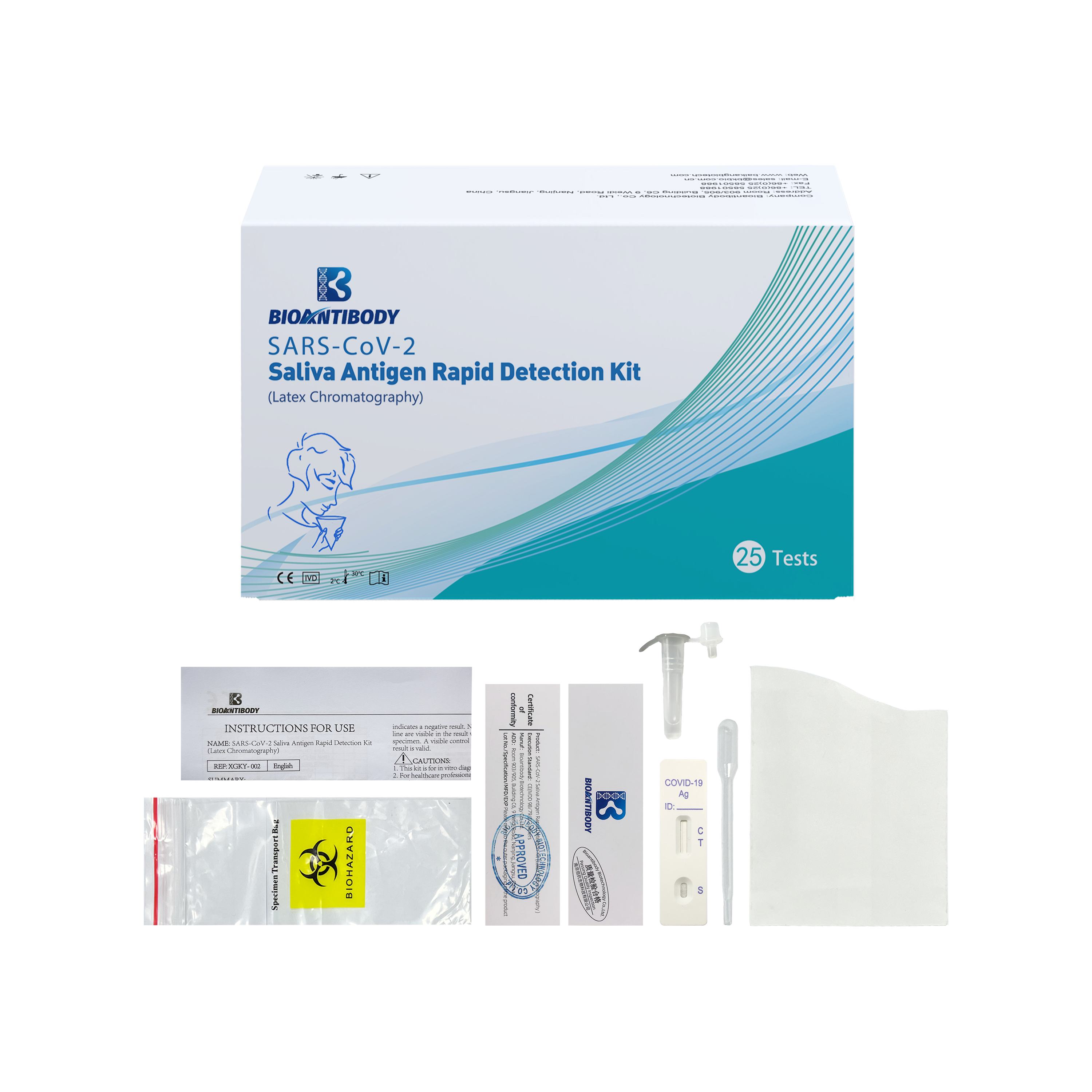
Components provided are listed in the table.
| Component/REF | XGKY-002 | XGKY-002-5 | XGKY-002-25 |
| Test Cassette | 1 test | 5 tests | 25 tests |
| Disposable paper cups | 1 piece | 5 pcs | 25 pcs |
| Sample Lysis Solution | 1 tube | 5 tubes | 25 tubes |
| Specimen Transport Bag | 1 piece | 5 pcs | 25 pcs |
| Instructions For Use | 1 piece | 1 piece | 1 piece |
| Certificate of Conformity | 1 piece | 1 piece | 1 piece |
Operation Flow

2. Optimal timing of specimen collection:After getting up and before brushing teeth, eating or drinking.

2 Take 200μL fresh saliva samples with a disposable dropper (The absorbed saliva rises to the first scale of disposable dropper) from container.
3 Transfer the saliva samples into the Extraction Tube and shake and mix it.
4 Firmly attach dropper lid to the top of the extraction tube. Then gently invert the extraction tube 5 times.
5 Transfer 3 drops (about 100μL) specimen to the sample well and start counting. Note: If using a frozen sample, the sample must be at room temperature before testing.


Result Interpretation

Positive Result
Colored bands appear at both test line (T) and control line (C). It indicates apositive result for the SARS-CoV-2 antigens in the specimen.
Negative Result
Colored band appear at control line (C) only. It indicates that the concentration of the SARS-CoV-2 antigens does not exist or below the detection limit of the test.
Invalid Result
No visible colored band appears at control line after performing the test. The
directions may not have been followed correctly or the test may have deteriorated. It is recommended that the specimen be re-tested.
Order Information
| Product Name | Cat. No | Size | Specimen | Shelf Life | Trans. & Sto. Temp. |
| SARS-CoV-2 Saliva Antigen Rapid Detection kit (Latex Chromatography) | XGKY-002 | 1test/kit | Saliva | 18 Months | 2-30℃ / 36-86℉ |
| XGKY-002-5 | 5 tests/kit | ||||
| XGKY-002-25 | 25 tests/kit |